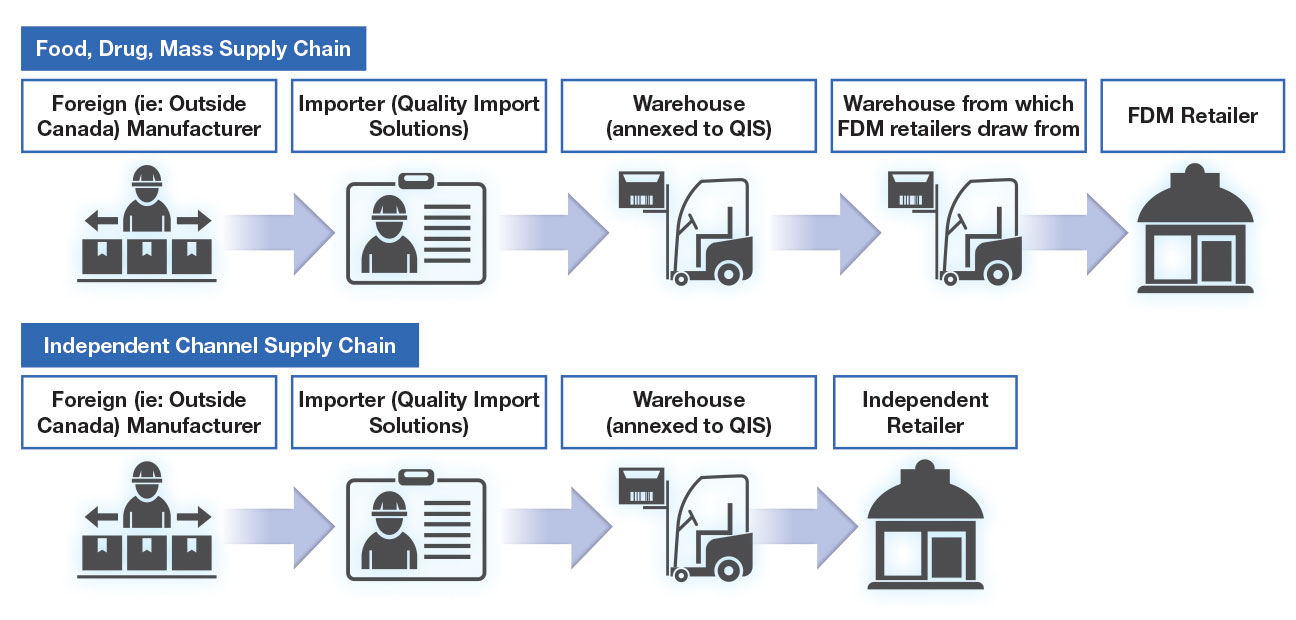
Hear From Our Amazing Clients
"Easy to get a hold of if we have questions and detailed in their release procedure, Quality IMPORT Solutions has been a pleasure to work with."

"We were using another third party importer and their costs to be prohibitive! Working with Quality IMPORT Solutions has been a refreshing alternative with a cost effective and simple process. Their friendly and knowledgeable team of individuals have helped us navigate through Canadian compliance topics with ease. We enjoy working with them!"

"With their simple, thorough procedures to check and clear our products into Canada quickly resolve issues and answer questions, Quality IMPORT Solutions is the ideal partner to be our importer of record."